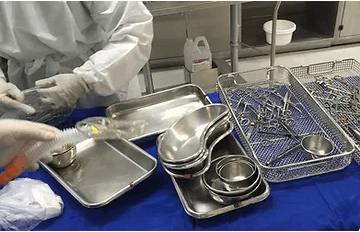
Hospitals are looking at automation systems to help with the inventory management of surgical trays, instrumentation kits, loaners, medical devices, and other hospital equipment.
Leveraging RFID in medical industry, institutions can automatically ensure the correct instrumentation sets and devices are available for each procedure within seconds instead of hours, and improve patient safety and outcomes.
What Are Surgical Instrument Tracking Systems For Hospitals?
An instrument tracking system is designed to improve on manual recording and paper trails for managing an institution’s instrument inventory at the tray level.
Hospitals are looking for solutions to common issues such as:
Procedures canceled due to incomplete sets or misplaced instruments.
Retained surgical instruments (RSI) incidents, when surgical items are left in a patient after the procedure has been completed.
Compliance with recommendations for instrument count in surgery.
Financial impact of instruments that are mistakenly discarded, lost or stolen.
Hospital-acquired infections (HAI) developed as a result of contaminated instruments and sterilization problems.
An instrument tracking system directly promotes surgical care safety and operational efficiency by assisting in inventory management and automating tracking at specific points of service, such as sterile processing, assembly, distribution, and in perioperative care.
Reprocessing Surgical Trays in SPD
Hospital SPD departments rely on an instrument tracking system to account for all surgical devices at various stages of decontamination, assembly, sterilization, and distribution.
SPD can track and identify every item delivered to them or returned from surgery prior to starting processing, with, for each, a comprehensive audit trail as well as IFUs (Instructions For Use).

The instrument tracking system assists the technicians in Assembly with building the instrument sets and trays, and documenting the reprocessing procedure for quality purposes.
Visual inspections are supplemented with lifecycle information regarding each instrument and device: How many cycles, item to be sent for repair, item loaned out… Additionally, when instruments are retired, all the information necessary for replacement is readily available.
Prior to sterilization, an SPD technician will scan the trays and containers to confirm all expected sets are accounted for. Any item missing would be highlighted in red on the screen of the handheld scanner until the discrepancy is corrected.
Once ready for distribution, a scanner will inform the SPD technicians of the exact instrument content of a set with its sterilization status, and secure its availability for a case scheduled.
Scheduling Surgical Trays and Devices for the OR
OR nurses will be able to confirm that all expected trays and instruments are available and that the procedure can go ahead as scheduled.
Completing a rapid, correct, and well-documented instrument count in surgery provides immeasurable patient safety improvements, as well as cost savings related to turnover time. Another component of instrument tracking systems allows non-scannable items to also be tracked in the OR suite for preference list building, counting, re-stocking of supplies, pricing, and billing purposes.
Accurate tracking of surgical assets and medical devices also plays a role in accreditation and compliance with government regulations.
For instance, in an effort to improve patient safety, the Joint Commission (JCAHO) developed the Universal Protocol to avoid wrong-site, wrong-procedure, and wrong-person surgery errors. Part of the protocol includes verifying the items and surgical sets required for the procedure using a standardized list.
Those items should be checked before and after the procedure to ensure that everything necessary is already in the operating room before the procedure starts, and to make sure that no surgical instrumentation is lost or left inside the patient after surgery.
To prevent these types of errors, all surgical items are recounted and inventoried after a procedure. If an item is missing, nurses must locate it before the procedure can be completed, at a cost of hundreds of dollars per minute of clinical time.
How to Build Instrument Tracking Systems for Hospitals?
RFID instrument tracking systems have been designed to solve instrument tracking and identification challenges and help healthcare organizations achieve automated, accurate surgical counts and billing for surgical services, as well as complete tracking of instrumentation. They are interoperable with an institution’s existing systems and meet UDI requirements.
The core of a surgical instrument tracking system lies in the unique RFID tagging of trays, containers, and other medical equipment.
Autoclavable trackers like the Xerafy Autoclavable RFID tags are qualified in the field to withstand repeated decontamination and sterilization cycles with no damage. Surgical instrument manufacturers such as Novo Surgical are developing smart surgical instruments that come enabled with the same tracking technology.
ROSWELL Autoclavable – An ultra-rugged tagging solution for all types of surgical trays and containers, this tag combines biocompatible materials with durability at the highest temperatures, optimizing workflow efficiency in the SPD.
MICRO Autoclavable – Compact and rugged, this tag is ideal for tracking all surgical trays, offers extended read range and high resistance to repeated reprocessing cycles.
MICRO Medical – Designed specifically for medical device manufacturers, this compact tag ensures seamless integration and traceability while maintaining compliance with sterilization standards.
The implementation of a surgical tracking system begins with attaching an RFID tag to each tray, container, and larger equipment. As each item is tagged and accounted for, a master list called the repository is created. Instrument lists for sets and trays are built from the repository. Instruments can be grouped together or listed separately with the required level of granularity. For example, the instrument list requirement for compliance with the Joint Commission can be fulfilled by including the correct number and generic type of instruments, rather than the specific instrument.
An easy-to-use handheld scanner is utilized to identify and view the sets and trays within seconds on a screen rather than only by an individual’s visual assessment. After scanning, the user can find additional information for each instrument in the operating room, decontamination area, hospital SPD, or throughout the facility as needed by multiple departments.
Institutions that are already deploying barcode systems will be able to use dual RFID-Barcode tracking equipment, making the transition to RFID simple and cost-effective.
Xerafy is a pioneer in Healthcare RFID, bringing to market several innovations that enable advanced identification and automation capabilities.
In addition to a complete range of field-proven RFID tags available off-the-shelf, Xerafy offers Custom RFID Tags services, covering everything from a personalization service bureau to custom-design engineering capabilities.